
Struggling to meet the stringent cleanliness standards for medical tubing? The microscopic world of surface imperfections can harbor bacteria, leading to patient risks and regulatory failures. Bright annealing1 offers a definitive solution, creating an ultra-clean, passive surface that fundamentally enhances safety and product quality.
Bright annealing enhances bio-clean surfaces by using a controlled, oxygen-free atmosphere to heat-treat stainless steel. This process creates an exceptionally smooth, chromium-oxide passive layer. This non-porous finish minimizes microbial adhesion, eliminates surface contaminants, and boosts corrosion resistance, making the tubing inherently cleaner and safer.
This isn't just about achieving a shiny finish; it's a critical manufacturing step that directly impacts patient safety and biocompatibility. For manufacturers of medical devices, understanding how this thermal process transforms a standard stainless steel tube into a bio-clean component is paramount. It’s the key to moving beyond simple surface cleaning to engineering inherent cleanliness directly into the material itself, a distinction that regulators and healthcare providers value highly.
In my years of experience at AKS Furnace, I've seen firsthand the challenges our clients in the medical device industry face. It's a world where precision is measured in microns and "good enough" is never acceptable. The shift from traditional mechanical polishing and chemical cleaning to advanced thermal processing like bright annealing represents a fundamental leap in quality. It addresses the root cause of contamination—surface topography and chemistry—rather than just treating the symptoms. This proactive approach not only simplifies downstream processes like sterilization but also builds a more robust, reliable, and inherently safer product from the ground up, a necessity in today's demanding healthcare environment.
What challenges exist in maintaining bio-clean surfaces in medical tubing?
You produce high-grade medical tubing, but the constant threat of microscopic surface flaws looms large. These imperfections can trap contaminants and foster biofilm growth, jeopardizing patient safety and regulatory compliance. Understanding these challenges is the first step toward creating truly sterile medical components.
The primary challenges in maintaining bio-clean surfaces on medical tubing include preventing microbial adhesion on microscopically rough surfaces, eliminating residual contaminants from manufacturing, and ensuring long-term corrosion resistance. Micro-pitting, grain boundaries, and surface oxides all create potential havens for contamination that are difficult to sterilize.
These challenges are not trivial; they represent the core obstacles that every medical tubing manufacturer must overcome. The first, microbial adhesion, is a direct function of surface topography. A surface that appears smooth to the naked eye can be a landscape of peaks and valleys at the microbial level, offering countless anchor points for bacteria to form biofilms. The second challenge, residual contaminants, is a byproduct of the manufacturing process itself. Drawing lubricants, polishing compounds, and microscopic debris can become embedded in the surface, resisting even the most aggressive cleaning protocols. Finally, the threat of corrosion undermines the very integrity of the material. A compromised surface not only releases metallic ions but also creates new pits and crevices, exacerbating the problem of microbial adhesion. For one of our clients, a producer of components for cardiovascular catheters, these issues led to inconsistent batch quality and costly delays in product validation. They learned that a reactive cleaning strategy was not enough; they needed a proactive method to create a fundamentally cleaner surface from the start. This realization is what begins the journey toward advanced thermal processing solutions.

In the medical device field, the term "clean" extends far beyond what is visible. The real battle is fought at the microscopic level, where the physical and chemical nature of a stainless steel surface dictates its interaction with biological systems. The challenges are multifaceted and interconnected, creating a complex problem that requires a sophisticated solution. Manufacturers must contend with the inherent topography of the metal, the residues left behind by fabrication, and the material’s long-term chemical stability in the human body. Overcoming these hurdles is not just a matter of quality control; it's a fundamental requirement for patient safety.
The Micro-Topography Battleground: Why Surface Finish is Paramount
The surface of stainless steel tubing, even after standard finishing processes, is far from perfect. Under a microscope, it reveals a complex topography of grain boundaries, micro-scratches, pits, and folds created during the tube drawing and forming processes. Each of these features increases the total surface area and creates ideal anchor points for microbes. A typical measure of this is the surface roughness average. While a mechanically polished tube might have, which feels smooth, this is still a rugged terrain for bacteria like Staphylococcus aureus or Pseudomonas aeruginosa, which measure only a few microns in size. These bacteria can easily lodge within these surface imperfections, initiating the formation of a biofilm—a protected colony of microbes that is notoriously resistant to sterilization.
A client of ours, "Medi-Tube Solutions," was struggling with this exact issue. They produced stainless steel cannulas and were facing recurrent biofilm formation despite a rigorous, multi-stage chemical cleaning and autoclave sterilization process. Upon analysis with scanning electron microscopy (SEM), we discovered that their manufacturing process left behind microscopic grooves and embedded particulate matter. These imperfections, invisible to the naked eye, were the source of their contamination issues. It became clear that no amount of post-process cleaning could fully compensate for a flawed initial surface. The solution had to involve refining the surface itself to make it inherently less hospitable to microbial life.
This highlights a critical principle: true bio-cleanliness cannot be achieved by cleaning alone. The surface itself must be engineered to resist contamination. This means minimizing the $R_a$ value and eliminating pits and crevices where bacteria can hide. It requires a process that can smooth the surface at a crystalline level, going far beyond the capabilities of mechanical abrasion or chemical etching, which can often introduce new, smaller-scale roughness.
The Hidden Danger of Manufacturing Residues
Every step in manufacturing, from drawing and welding to cutting and polishing, introduces foreign substances to the tubing's surface. Drawing lubricants, typically oil- or wax-based, are essential for the forming process but are notoriously difficult to remove completely. If not fully eliminated before heat treatment, these hydrocarbons can carbonize on the surface, creating a tenacious, dark residue that is not only a contaminant itself but also a source of pitting and corrosion. Similarly, abrasive particles from grinding or polishing can become embedded in the soft steel, creating sites for galvanic corrosion and microbial attachment.
Consider the typical production workflow and the contamination risks at each stage:
| Manufacturing Step | Primary Contaminant Risk | Impact on Bio-Cleanliness |
|---|---|---|
| Tube Drawing/Forming | Drawing Lubricants (Oils, Waxes) | Carbonization during annealing, creating surface defects and harboring bacteria. |
| Seam Welding | Oxides, Weld Spatter | Creates a rough, chemically different zone that is prone to corrosion. |
| Mechanical Polishing | Abrasive Particles, Polishing Compounds | Embeds particles into the surface, creating sites for galvanic corrosion. |
| Laser Cutting | Metallic Debris, Slag | Leaves sharp edges and melted, re-solidified material that can trap cells. |
| Chemical Cleaning | Residual Acids, Detergents | Can cause micro-etching or leave a chemical film that interferes with biocompatibility. |
The challenge is that these residues are often locked into the surface's micro-topography. A simple solvent wash may remove the bulk of the lubricant, but a thin film can remain in the valleys of the surface roughness. This is why a comprehensive cleaning protocol, such as multi-stage ultrasonic vapor degreasing, is a mandatory precursor to any thermal process. For "Medi-Tube Solutions," we helped them identify that their pre-cleaning process was insufficient, leaving behind a molecular film of lubricant that compromised their subsequent annealing efforts.
Corrosion: The Silent Saboteur of Biocompatibility
Stainless steel's "stainless" property is derived from a very thin, invisible, and self-healing passive layer of chromium oxide ($Cr_2O_3$) on its surface. This layer protects the underlying iron from reacting with its environment. However, this layer is fragile. Mechanical damage (scratches), chemical attack (from chlorides or acids), or high temperatures in an uncontrolled atmosphere can compromise it. When this passive layer breaks down, micro-corrosion begins. This process not only damages the surface, creating more pits and crevices for bacteria, but it can also leach metallic ions like nickel, chromium, and iron into the surrounding environment.
In the context of medical devices, this is a critical failure. The leaching of metallic ions can trigger allergic reactions, cause inflammation, and lead to cytotoxicity, a failure of ISO 10993 biocompatibility testing. A compromised passive layer turns a safe, inert material into a reactive one. Many manufacturers don't realize that their standard annealing process, if performed in open air, actually creates a thick, unstable iron-rich oxide scale. This scale must then be removed with aggressive pickling acids, a process that can unevenly etch the surface and fail to restore a high-quality passive layer, leaving the tubing vulnerable to corrosion.
This is where the choice of annealing technology becomes a make-or-break decision. Maintaining and enhancing this passive layer is the ultimate goal. A process is needed that not only cleans and smoothes the surface but also chemically engineers the ideal passive layer as a final, integral step. This is the fundamental challenge that bright annealing is uniquely positioned to solve.
Bright annealing creates a passive chromium-oxide layerTrue
The oxygen-free environment during bright annealing promotes formation of a protective chromium-oxide layer that enhances corrosion resistance and biocompatibility.
Mechanical polishing eliminates all surface imperfectionsFalse
While mechanical polishing improves surface smoothness, it can't eliminate microscopic pits and grain boundaries that harbor bacteria.
How does bright annealing address these challenges in stainless steel tubing?
The myriad challenges of surface contamination can feel insurmountable, with each manufacturing step adding another layer of risk. Traditional methods often fall short, leaving you worried about product quality and patient safety. Bright annealing offers a comprehensive solution by fundamentally altering the steel's surface for superior cleanliness.
Bright annealing addresses these challenges by heat-treating stainless steel in a controlled, oxygen-free atmosphere, typically high-purity hydrogen. This prevents oxidation, vaporizes residual surface contaminants, smoothes the surface through recrystallization, and reforms a robust, chromium-rich passive layer that is highly resistant to corrosion and microbial adhesion.
Moving from recognizing the problems to implementing a solution requires a paradigm shift in how we approach surface treatment. Instead of viewing cleaning as a separate, corrective action, bright annealing integrates surface purification and enhancement directly into the metallurgical process. It's not simply "heating metal"; it is a precise, scientifically controlled procedure that tackles all three major challenges—topography, contaminants, and corrosion—simultaneously. At our facility, when we guide a client through the process, we emphasize that they are not just buying a furnace; they are adopting a new quality philosophy. The magic happens within the furnace's controlled atmosphere, where the tubing is transformed. The process relieves the internal stresses from manufacturing, which helps to flatten the micro-topography. It vaporizes residual organic compounds, and most importantly, it rebuilds the chromium oxide passive layer from the inside out, creating a surface that is not just clean but truly bio-inert. This transition from a reactive to a proactive quality strategy is what sets leading medical device manufacturers apart.

Bright annealing is a term that describes a specific category of thermal processing defined by its outcome: a bright, oxide-free surface. This is achieved by carefully controlling the atmosphere within the furnace during the heating and cooling cycle. This control is the cornerstone of the process and is what allows it to so effectively address the challenges of creating a bio-clean surface. Unlike open-air annealing, which is a crude, destructive process from a surface-quality perspective, bright annealing is a highly refined, constructive one. It systematically purifies, smoothes, and passivates the stainless steel surface at a molecular level.
The Science of Controlled Atmospheres: Preventing Oxidation and Vaporizing Contaminants
The defining feature of a bright annealing furnace, like our AKS continuous bright annealing lines2, is its ability to maintain an atmosphere completely devoid of oxygen. This is typically achieved using a continuous flow of high-purity (99.99% or higher) cracked ammonia or, for the most demanding medical applications, pure hydrogen. The furnace chamber, or muffle, is engineered to be perfectly gas-tight to prevent any air from leaking in. Crucially, the dew point of the atmosphere—a measure of its moisture content—is kept extremely low, often below -60°C. This is because at annealing temperatures (typically 1040-1150°C for austenitic stainless steels), any oxygen or water vapor would instantly react with the steel's surface elements.
In this oxygen-free environment, two critical things happen. First, oxidation is completely prevented. Instead of forming a thick, flaky iron oxide scale, the surface remains pristine and bright. This eliminates the need for post-annealing acid pickling, a harsh chemical process that creates its own set of contamination risks and environmental hazards. Second, the combination of high temperature and a reactive hydrogen atmosphere effectively vaporizes and removes any residual organic contaminants, like traces of drawing lubricants. The hydrocarbons are cracked and reduced, leaving behind a purely metallic surface ready for the next stage of transformation. This is a level of cleaning that no solvent-based system can achieve.
At AKS, our furnaces are equipped with real-time gas analyzers and dew point sensors3 to ensure the atmosphere integrity is maintained throughout the entire process. This precision control is non-negotiable for medical-grade applications. It guarantees that every inch of tubing emerges from the furnace in a state of ultimate purity, free from both organic residues and metallic oxides.
Recrystallization and Surface Smoothing
The mechanical stress induced during the tube drawing process creates a distorted and hardened internal grain structure. The primary purpose of annealing is to reverse this by heating the steel above its recrystallization temperature. At this point, new, strain-free grains begin to form, restoring the metal's ductility and softness. This metallurgical reset is well-known, but a key secondary benefit of bright annealing is its impact on the surface topography. As the crystal structure rearranges itself into a lower-energy state, there is a subtle but significant smoothing effect at the microscopic level.
This process, known as thermal smoothing, reduces the peaks and valleys on the surface, measurably lowering the $R_a$ value. While it won't remove deep scratches, it refines the overall surface texture, making it less conducive to bacterial attachment. When we analyzed tubing for "Medi-Tube Solutions" before and after processing in our bright annealing furnace, SEM images showed a clear reduction in micro-roughness and a more uniform surface profile. This physical change is a direct result of the controlled thermal cycle. The precise multi-zone heating and advanced cooling systems in our furnaces ensure this recrystallization happens uniformly, not just through the cross-section of the tube wall but also along its entire length, guaranteeing consistent properties and surface quality.
Reforming the Passive Layer: The Key to Bio-Inertness
This is arguably the most critical benefit of bright annealing for medical applications. The passive layer is the key to stainless steel's corrosion resistance and biocompatibility. In a high-temperature, low-dew-point hydrogen atmosphere, a fascinating chemical selection process occurs on the surface of the steel. The atmosphere is reducing enough to prevent iron and nickel from oxidizing, but it allows the most reactive element, chromium, to selectively combine with the trace amounts of oxygen present to form a new passive layer.
This newly formed layer is not like the thick, iron-rich scale from open-air annealing. Instead, it is an extremely thin (typically 2-3 nanometers), dense, and highly uniform layer composed almost entirely of chromium oxide ($Cr_2O_3$). This specific oxide is incredibly stable, non-reactive, and self-healing, providing the highest possible level of corrosion resistance. It effectively seals the underlying metal from the external environment, preventing the leaching of metallic ions and providing a smooth, bio-inert surface.
For our client "Medi-Tube Solutions," achieving this high-integrity passive layer was the final piece of the puzzle. Validated through X-ray Photoelectron Spectroscopy (XPS), the surface chemistry of their bright-annealed tubes showed a significantly higher chromium-to-iron ratio compared to their old process. This data became a cornerstone of their regulatory submission, providing concrete evidence of a superior, safer product. By engineering the ideal passive layer, bright annealing transforms the steel tube into a component that is passive and clean by design.
Bright annealing prevents oxidationTrue
Bright annealing uses an oxygen-free atmosphere to prevent oxidation, keeping the steel surface pristine.
Bright annealing requires acid picklingFalse
Unlike traditional methods, bright annealing eliminates the need for acid pickling by preventing oxidation in the first place.
What impact does bright annealing have on the quality and cleanliness of medical tubing?
You need to demonstrate that your medical tubing isn't just compliant, but superior. But how do you quantify "clean" and "high-quality" in a way that resonates with regulators and builds customer trust? Bright annealing delivers measurable improvements that set your products apart.
Bright annealing has a profound impact on medical tubing quality by creating an ultra-smooth, non-porous surface that significantly reduces microbial adhesion. It enhances corrosion resistance for better biocompatibility, eliminates post-annealing chemical cleaning, and ensures a visually bright, uniform finish that signals superior cleanliness and process control.
The benefits of bright annealing are not abstract; they are tangible, measurable improvements that directly address the most critical quality metrics for medical components. When a manufacturer adopts this technology, they see a transformation across their entire quality control spectrum. The impact begins with a quantifiable reduction in surface roughness, which can be directly correlated to a lower risk of biofilm formation—a key selling point for any device used in sterile environments. This is complemented by a dramatic improvement in corrosion resistance4, which is essential for ensuring the device remains inert and safe throughout its lifecycle. Furthermore, the process simplifies the entire production chain. By producing a tube that is already clean and passivated, it eliminates entire steps of hazardous chemical processing, reducing costs, improving worker safety, and removing potential sources of contamination. For my clients, the bright, mirror-like finish becomes more than just an aesthetic feature; it becomes a hallmark of quality, a visual promise of the microscopic purity that lies on the surface.
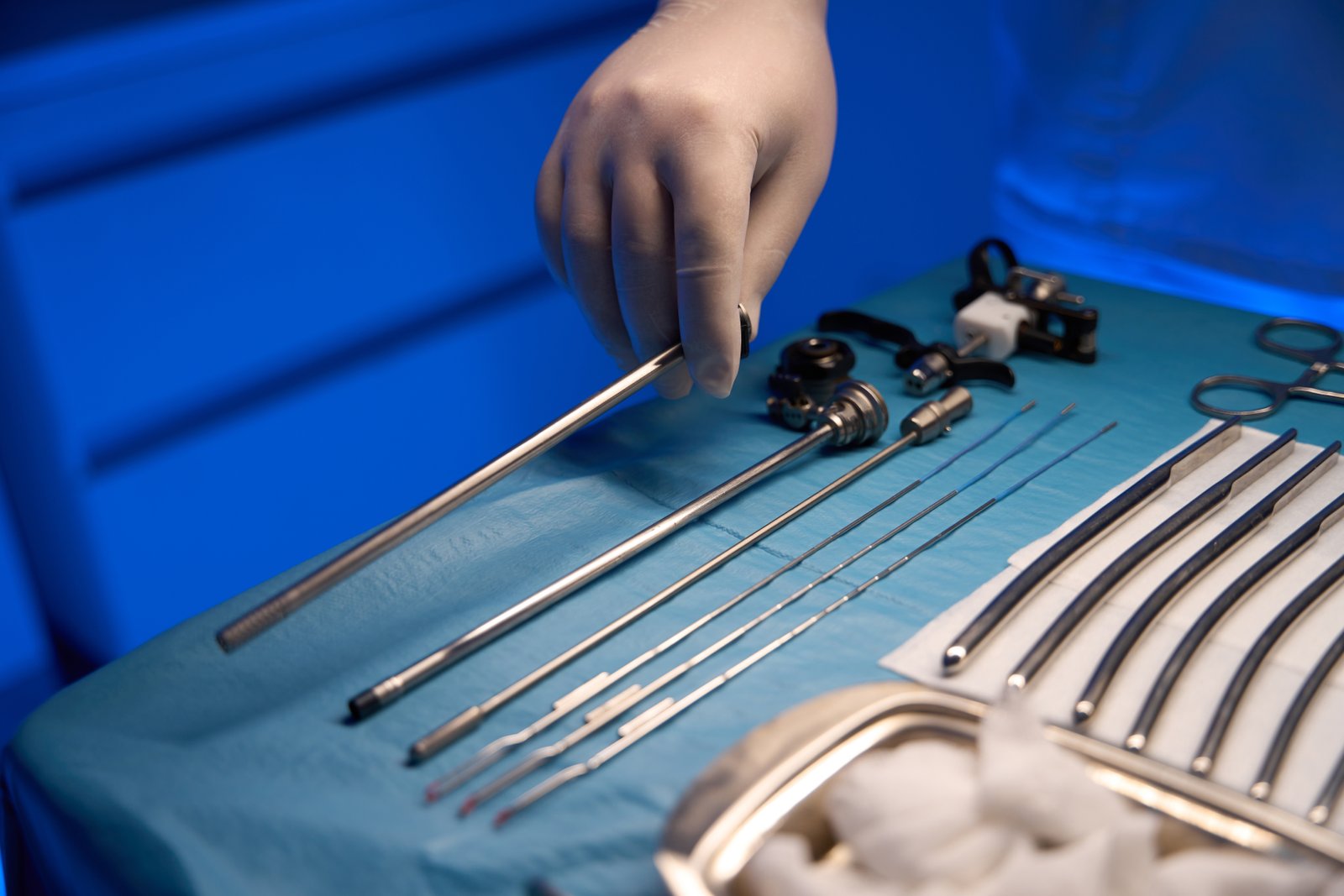
The adoption of bright annealing is a strategic decision that elevates the quality and cleanliness of medical tubing from a baseline requirement to a competitive advantage. The impact is multifaceted, affecting the physical, chemical, and biological properties of the final product. These improvements are not merely incremental; they represent a fundamental shift in what is achievable in terms of surface perfection. By moving the focus from post-process correction to in-process optimization, manufacturers can achieve a level of quality that is simply unattainable with older methods, and they can prove it with hard data.
Quantifiable Reduction in Surface Roughness and Microbial Adhesion
The most immediate and demonstrable impact of bright annealing is on the surface topography. As the metal recrystallizes in a controlled environment, the surface becomes measurably smoother. For a typical 316L stainless steel medical tube, a standard drawn and polished surface might have a roughness average of 0.4-0.6 µm. After being properly bright annealed in an AKS furnace, it's common to see this value drop to below 0.3 µm, with some processes achieving even finer finishes. While this may seem like a small number, its biological implication is enormous.
Numerous studies in microbiology have shown a direct correlation between surface roughness and the rate of bacterial adhesion and biofilm formation. Smoother surfaces offer fewer anchor points for microbes to attach and colonize. A reduction in from 0.5 µm to 0.3 µm can result in a significant reduction in bacterial load after exposure. For instance, a client producing surgical instruments was able to use this data to validate a more efficient sterilization cycle, as the smoother surface was easier to sterilize completely. This data, generated from profilometers and confirmed with SEM imaging, provides powerful, quantitative proof of a cleaner product. It changes the conversation from "we clean our tubes" to "our tubes are engineered to stay clean."
Enhanced Corrosion Resistance and Biocompatibility
The quality of the passive layer is the single most important factor for biocompatibility. A poorly formed, iron-rich, or damaged passive layer will fail in the corrosive environment of the human body, leading to the release of cytotoxic ions. The thin, dense, chromium-oxide layer created during bright annealing provides a level of corrosion resistance that far surpasses that achieved by chemical passivation. This can be verified using standardized tests. For example, when subjected to a neutral salt spray test (ASTM B117), properly bright annealed tubes can often withstand hundreds of hours without any signs of rust, whereas a merely passivated tube might show signs of corrosion much sooner.
This superior corrosion resistance directly translates to better biocompatibility. The stable passive layer prevents the metal from reacting with bodily fluids, ensuring it passes critical ISO 10993 tests for cytotoxicity, sensitization, and irritation. When "Medi-Tube Solutions" switched to our bright annealing process for their catheter guidewires, they not only eliminated batch failures in cytotoxicity testing but were also able to provide their customers with corrosion test data that certified the long-term stability of their product. This chemical inertness is the true definition of a bio-clean surface and a critical requirement for any implantable or fluid-path component.
Eliminating Post-Processing Contamination Risks
A frequently overlooked impact of bright annealing is its ability to streamline the manufacturing process and eliminate sources of contamination. The traditional "anneal and pickle" workflow is fraught with risk. Open-air annealing creates a thick oxide scale that must be removed. This is done by immersing the tubes in a hazardous bath of nitric and hydrofluoric acid. This pickling process is difficult to control; it can over-etch the surface, increasing roughness, or it can be incomplete, leaving behind patches of scale. Most importantly, it introduces a significant risk of residual acid being trapped in the microscopic pores of the surface, which can cause downstream quality problems.
Bright annealing makes this entire step obsolete. The tubing emerges from the furnace bright, clean, and already passivated. There is no scale to remove. This has a massive positive impact:
- Improved Cleanliness: An entire chemical contamination step is removed from the process.
- Enhanced Safety: Workers are no longer exposed to dangerous acids.
- Environmental Benefits: Eliminates the need to treat and dispose of hazardous pickling waste.
- Cost Reduction: Saves money on chemicals, waste disposal, and the floor space required for pickling lines.
By creating a finished surface directly within the furnace, bright annealing provides a cleaner, safer, and more efficient path to a superior product. It shortens the production cycle and builds quality into the process, rather than trying to inspect or clean it in later.
Bright annealing reduces surface roughnessTrue
Bright annealing creates an ultra-smooth surface, typically reducing roughness from 0.4-0.6 µm to below 0.3 µm, which significantly decreases microbial adhesion.
Pickling is required after bright annealingFalse
Bright annealing eliminates the need for pickling as the tubing emerges from the furnace already clean and passivated, removing a major contamination risk.
What solutions can manufacturers employ to ensure bio-clean surfaces through bright annealing?
You understand that bright annealing is the key, but knowing the goal is different from achieving it consistently. The wrong equipment or an uncontrolled process can lead to subpar results, wasting time, energy, and material. Focusing on specific furnace technologies ensures bio-clean surfaces every time.
To ensure bio-clean surfaces, manufacturers must employ bright annealing furnaces with exceptionally precise atmosphere and temperature control. Key solutions include using high-purity hydrogen atmospheres, maintaining a very low dew point to prevent oxidation, and utilizing advanced, rapid cooling systems to preserve the smooth, stress-free finish.
The successful implementation of bright annealing for medical-grade products hinges entirely on the quality and capability of the furnace. This is not a process where "close enough" is acceptable. Minor fluctuations in temperature, a small air leak in the muffle, or an elevated dew point can ruin an entire production run, resulting in oxidized, dull, or dimensionally inconsistent tubing. Therefore, the solution for manufacturers lies in investing in equipment that provides unwavering process stability and precision. When I consult with companies entering the medical device market, I always emphasize that the furnace is not a cost center; it is the heart of their quality assurance program. It is the tool that empowers them to deliver on the promise of a bio-clean surface. At AKS, our design philosophy is built around this principle. We engineer systems—from the gas delivery and monitoring to the muffle construction and cooling zones—that provide the rigorous control necessary to meet and exceed the standards for medical applications. The solution is to choose a furnace that is designed from the ground up for precision, not one that has been retrofitted or adapted.

Achieving a perfect, bio-clean surface through bright annealing is a science that demands the right tools. A manufacturer's success is directly tied to the technological sophistication of their annealing line. It requires a holistic system where every component is designed to contribute to a stable, pure, and precisely controlled environment. Investing in the right technology is the most critical step a manufacturer can take to guarantee consistent, high-quality results for demanding medical applications. This involves a deep look into the furnace's atmosphere control and thermal profiling capabilities5, and physical design.
The Critical Role of Atmosphere Control and Purity
The essence of bright annealing is the atmosphere. For medical-grade stainless steel, the industry benchmark is high-purity, dry hydrogen. Hydrogen is a powerful reducing agent, meaning it actively scavenges for any stray oxygen atoms, preventing them from reacting with the steel. The purity of this gas is paramount. An inferior gas supply can introduce contaminants that deposit on the tubing surface. The second, equally critical parameter is the dew point. Water vapor ($H_2O$) is a potent oxidizing agent at high temperatures. Therefore, the furnace atmosphere must be kept exceptionally dry, with a dew point consistently below -60°C.
To achieve this, manufacturers need a furnace with a sophisticated gas management system. This includes:
- High-Quality Gas Source: Using electrolytic-grade hydrogen or an on-site high-purity generator.
- Precision Flow Control: Mass flow controllers to ensure a stable, positive pressure inside the furnace, preventing air ingress.
- Real-Time Monitoring: Continuous monitoring of the furnace atmosphere's oxygen content (in parts per million) and dew point. Our AKS furnace systems incorporate these sensors with alarm triggers and data logging, providing a complete record of the process conditions for every batch, which is invaluable for quality audits and validation.
Choosing a furnace with a robust, integrated atmosphere control system is the number one solution for preventing the microscopic oxidation that undermines a bio-clean surface.
Precision in Thermal Profiling: Heating and Cooling
Achieving the desired metallurgical properties and surface finish requires a precise thermal journey. This isn't just about reaching a peak temperature; it's about controlling the entire heating and cooling curve. The furnace must have multiple, independently controlled heating zones. This allows the operator to create a specific temperature profile that gently heats the tubing6 to the annealing temperature, holds it there for the exact time needed for full recrystallization (soaking), and then begins the cooling process. Uneven heating can lead to incomplete annealing or grain growth, affecting both mechanical properties and surface consistency.
The cooling phase is just as critical. The tubing must be cooled rapidly but in a controlled manner, all while remaining in the protective atmosphere until it is well below the oxidation temperature. Our "Advanced Cooling System" uses a sealed, water-jacketed cooling muffle combined with forced convection of the protective atmosphere. This rapid cooling "locks in" the fine-grained microstructure and the pristine surface finish achieved in the heating zone. Slow, uncontrolled cooling can lead to sensitization (carbide precipitation at grain boundaries), which severely degrades corrosion resistance, or discoloration of the surface, even in a good atmosphere. A furnace that provides precise control over the entire thermal cycle is essential for achieving repeatable, high-quality results.
Furnace Design and Material Handling
The physical construction of the furnace plays a vital role. The heart of a continuous bright annealing furnace is the muffle—the sealed tube or chamber through which the product and the protective atmosphere travel. For the high temperatures required for stainless steel, this muffle must be made from a high-nickel alloy, such as Inconel or similar materials, that can withstand the heat without degrading or releasing contaminants. The muffle's design, especially its seals at the entry and exit points, must be flawlessly engineered to remain gas-tight7. Even a pinhole leak can compromise the entire process.
Furthermore, how the tubing is transported through the furnace is a key consideration. For smaller diameter tubing or individual parts, a Mesh Belt Furnace is often an ideal solution. The belt itself must be made of a suitable alloy and have a weave that supports the product without causing scratches or marks. For continuous tubing (coil-to-coil), the furnace is designed as a straight-through line. In all cases, the internal surfaces of the furnace and the transport mechanism must be designed to be non-reactive and non-abrasive. This ensures the pristine, bright surface created during the process is not mechanically damaged before it exits the furnace. Investing in a furnace built with high-quality materials and thoughtful design is a long-term solution for reliable production.
Hydrogen prevents oxidationTrue
High-purity hydrogen acts as a reducing agent, scavenging oxygen to prevent surface oxidation during bright annealing.
Slow cooling improves finishFalse
Controlled rapid cooling is essential to lock in microstructure and surface finish; slow cooling causes sensitization and discoloration.
What are the best practices for utilizing bright annealing in medical tubing production?
You have the right furnace, but technology alone is not enough. Best practices are what separate average results from exceptional, certifiable quality. Small deviations in your process can compromise the very bio-clean surface you worked so hard to achieve, undermining your entire investment.
Best practices include meticulous pre-cleaning of medical tubing to remove all lubricants before annealing. This is followed by strict, documented control of furnace parameters like atmosphere purity and dew point. ly, the process must be validated using surface analysis techniques like SEM and corrosion testing to ensure consistent, reliable results.
Implementing best practices transforms bright annealing from a simple heating step into a robust, repeatable manufacturing science. It’s about building a culture of precision around the process. In my experience, the most successful medical device manufacturers are those who treat their annealing line with the same respect as a cleanroom. They understand that the quality of the final product is determined by the discipline they apply at every stage. This begins long before the tubing ever enters the furnace and continues through rigorous post-process validation. Adopting these best practices is not about adding complexity; it's about eliminating variables and controlling the process so tightly that a perfect outcome is not just possible, but predictable. It’s this predictability and documented control that satisfies auditors, builds customer confidence, and ultimately protects patients. This disciplined approach ensures that the advanced capabilities of your furnace are fully realized, run after run.

Owning a high-performance furnace is the first step; operating it to its maximum potential requires a disciplined, systematic approach. The best practices for bright annealing in medical tubing production revolve around three core principles: pristine preparation, rigorous process control, and empirical validation. These practices create a framework that ensures the theoretical benefits of the technology are consistently translated into tangible, high-quality products. For any manufacturer aiming for the top tier of the medical device market, these practices are non-negotiable.
Pre-Annealing Preparation: The Foundation of Cleanliness
The most advanced furnace in the world cannot fix a poorly prepared product. The single most common failure point in bright annealing is inadequate pre-cleaning. Any residual drawing lubricants, oils, or shop dust left on the tubing will vaporize, decompose, or carbonize at high temperatures. This can lead to a host of problems: a sooty or discolored surface, carburization of the steel (making it brittle), and contamination of the furnace's internal components and atmosphere. The mantra must be: the surface must be surgically clean before it enters the furnace.
A best-in-class pre-cleaning protocol typically involves a multi-stage process with ultrasonic cleaning8. This often starts with an alkaline wash to saponify the bulk of the oils, followed by multiple rinse stages. The final, critical step is often an ultrasonic cleaning cycle in a solvent vapor degreaser. This method uses high-frequency sound waves to create cavitation bubbles that scrub the surface at a microscopic level, dislodging contaminants from the deepest pores and scratches. The part then emerges from the vapor zone perfectly clean and dry. Operators should wear clean, lint-free gloves when handling parts post-cleaning to prevent re-contamination. Instituting and auditing a rigorous pre-cleaning protocol is the most important best practice for achieving a flawless bright annealed finish.
Process Validation and Continuous Quality Control
"Set it and forget it" is not a viable strategy in medical manufacturing. A best practice is to move from simple process monitoring to active process validation. This means proving, with objective data, that your bright annealing process consistently produces a surface with the required characteristics. This involves a formal validation plan (IQ/OQ/PQ - Installation/Operational/Process Qualification) for the furnace itself. On an ongoing basis, it means regularly sampling and testing the product.
Essential QC testing should include:
- Surface Roughness Testing: Using a profilometer to verify that the $R_a$ value is within the specified limits.
- Visual Inspection: Checking for brightness, color consistency, and the absence of soot or haze under magnification.
- Corrosion Testing: Periodically subjecting samples to salt spray or other corrosive environments to validate the integrity of the passive layer.
- Microscopic Analysis: Using Scanning Electron Microscopy (SEM) on a sample basis to visually inspect the surface for micro-defects and confirm a smooth, clean topography.
For our client "Medi-Tube Solutions," we helped them establish a QC protocol where they pull a sample from the beginning and end of each coil and run it through a series of these tests. The data is logged against the furnace's process data, creating a complete traceability record for each production run. This level of documentation is invaluable for regulatory compliance and for quickly diagnosing any process deviations.
Furnace Maintenance and Operational Discipline
A high-performance furnace is a precision instrument that requires regular, disciplined maintenance to perform at its peak. A proactive maintenance schedule is a critical best practice that prevents costly downtime and quality escapes. This schedule should be developed in partnership with the furnace manufacturer (like AKS) and should become a routine part of plant operations.
Key maintenance activities include:
- Atmosphere System Integrity Checks: Regularly performing leak checks on the muffle and all gas plumbing using a helium leak detector or similar device. A small leak can go unnoticed but have a major impact on product quality.
- Sensor Calibration: The thermocouples, oxygen analyzers, and dew point sensors that control the process must be calibrated on a regular schedule to ensure their accuracy. Drifting sensors can lead to an out-of-spec process.
- Muffle and Belt Inspection: Regularly inspecting the condition of the furnace muffle and mesh belt (if applicable) for signs of wear, warping, or contamination.
- Record Keeping: Maintaining detailed logs of all maintenance activities, calibration results, and any operational issues.
This operational discipline ensures the furnace continues to perform as designed, day in and day out. It fosters a sense of ownership among the operators and technicians and reinforces the understanding that bright annealing is a precise, scientific process, not just another piece of factory machinery.
Pre-cleaning is critical for bright annealingTrue
The text emphasizes that inadequate pre-cleaning is the most common failure point, as contaminants can lead to surface defects and furnace contamination.
Furnace maintenance is optionalFalse
The text clearly states that proactive maintenance is a critical best practice, including regular checks of atmosphere systems, sensor calibration, and muffle inspections.
Conclusion
Ultimately, bright annealing is not just a finishing process but a foundational technology for medical manufacturing. It proactively engineers an ultra-smooth, chemically pure, and robustly passive surface. This ensures superior biocompatibility, reduces contamination risks, and delivers the quality and safety that modern medical devices demand.
-
Learn why bright annealing is crucial for tube cleanliness and biocompatibility ↩
-
Explore the specialized features of AKS bright annealing lines for superior steel processing quality ↩
-
Learn about the role of dew point sensors in maintaining furnace atmosphere integrity ↩
-
Learn why corrosion resistance is crucial for biocompatibility in medical devices ↩
-
Learn the technologies that ensure stable and precise environmental conditions for annealing processes. ↩
-
Understand the impact of temperature control on the quality and consistency of stainless steel products. ↩
-
Explore the importance of muffle integrity in maintaining protective atmospheres during annealing. ↩
-
Understand the role of ultrasonic cleaning in achieving a clean finish for annealing ↩


